Understanding the Regulatory Barriers to Hemp in Animal Feed
Author: Dr Bronwyn Blake, June 2024
In November 2017 Food Standards Australia New Zealand allowed hemp seed products to legally be consumed by humans. It was a tremendous step forward for the Australian hemp industry.
Unfortunately, many of us automatically assumed that if something was deemed safe for humans, it could also be fed to animals, including pets. As a result, businesses and products featuring hemp for animals started popping up all over the country. The problem was that many businesses were making health claims, which is not allowed for an unregistered product or ingredient.
This naivety was the catalyst for today’s restrictions. We can safely assume that compliant pet food manufacturers were a little put out by all of the non-compliant hemp products hitting the market and some of the health claims that accompanied them. What we are quickly learning as an industry is that, no matter what the science says, you can’t make a claim unless it has already been approved by the government.
In August 2023, the Australian Pesticides and Veterinary Medicines Authority (APVMA) published their position on cannabis, stating that they consider all products containing cannabis (including hemp) or cannabinoids as Veterinary Chemical Products (Veterinary Medicines) that require registration.
Understanding the regulators
To begin addressing this issue, we first need to understand where hemp fits into different government regulations.
As shown in Figure 1., to fall under the APVMA’s jurisdiction, a product or ingredient must have therapeutic properties. APVMA representatives have confirmed that both cannabinoids and omega 3 & 6 are active constituents with therapeutic properties and that is why hemp has been identified as a veterinary chemical product.
Furthermore, hemp seed and its derivatives, other than hemp protein, do not appear on any of the Generally Recognized as Safe (GRAS) lists included in the AgVet Code, which explains why hemp as an ingredient is currently non-compliant.
Clearly, some byproducts of hemp seed processing, such as hemp seed meal and hemp hulls, are used solely as sustenance products and should be regulated by the States and Territories (see Figure 1). For example, hemp hulls provide fibre, and hemp seed meal offers both protein and fibre. When used exclusively as sustenance ingredients, these products should not be governed by the APVMA. However, the APVMA claims it has not received sufficient information to determine whether these ingredients are non-therapeutic and truly sustenance only.
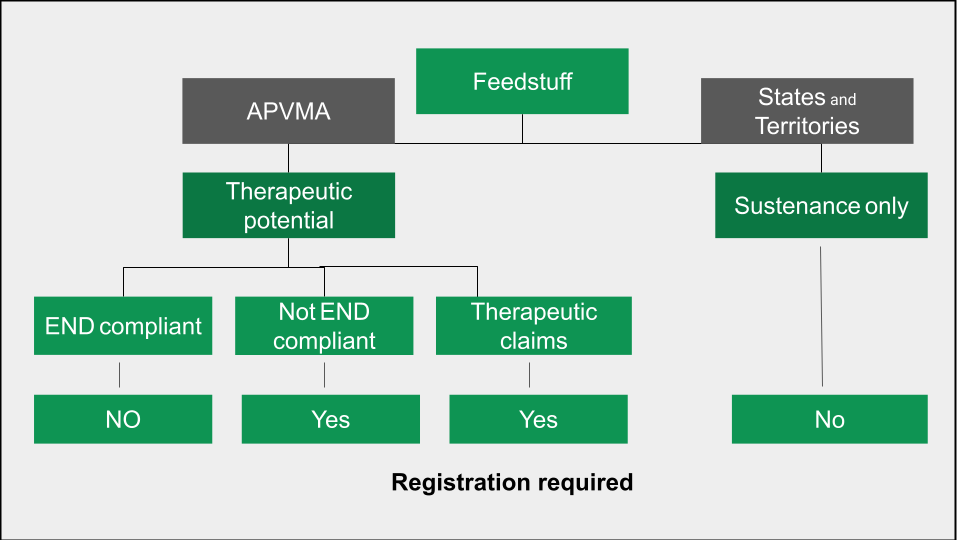
Hemp seed oil with its wonderful omega profile definitely comes under the therapeutic banner. There are a few pathways for these types of products. If it is being sold as a feed ingredient without therapeutic claims and is END compliant it does not require registration.
What is END compliance?
END refers to Excluded Nutritional or Digestive (END) products. Products or ingredients with END classification, are feeds rather than medicines, and are consumed voluntarily by the animal, do not require registration with APVMA. To be classified as an END product, hemp seed and its byproducts must be listed on one of the Generally Recognised as Safe (GRAS) lists stated in the AgVet code, or be declared safe via an Ingredient Determination. Currently, hemp seed and its derivatives (except hemp protein) do not appear on any AgVet Code approved GRAS lists and so require registration.
Below are additional requirements for END compliance:
- Ingredients: the product must not contain certain ingredients, such as antibiotics; and all ingredients must be on at least one of the specified list of substances that are generally recognised as safe (the ‘GRAS’ lists).
- Manufacturing systems: the product must be made to one of a number of specified quality assurance (QA) systems.
- Labelling: the label must contain specified information about the product.
- Claims: the label does not include a claim that the product treats a disease, condition or injury, unless it is supplied by or in accordance with instructions of a vet and any claim is backed up by high quality scientific evidence.
If you are manufacturing animal feeds and NOT following the above you are likely not compliant and risking both your business and the integrity of the Australian Hemp Industry.
Industry activity to date
Since the APVMA announced their position on cannabis in August 2023, the AHC has engaged in a number of meetings both with APVMA and the Federal Department of Agriculture, Forestry and Fisheries (DAFF).
On 29 November 2023 the AHC Fodder Group hosted a meeting between key industry stakeholders and representatives of the APVMA. The meeting was run as a Q&A session, so we, as an industry, could gain clarity around what was required to become compliant. We put a series of questions to the APVMA and their written responses can be found here.
The next course of action was to send a letter to Federal Agricultural Minister, Minister Watt. The letter requested:
- Hemp and its derivatives were officially acknowledged as Animal Feed Products, rather than Veterinary Chemical Products.
- Permit the use of hemp seeds and their derivatives, to allow businesses to continue operating, while working with APVMA to achieve compliance.
The Minister’s response was simply to follow the instruction of the APVMA.
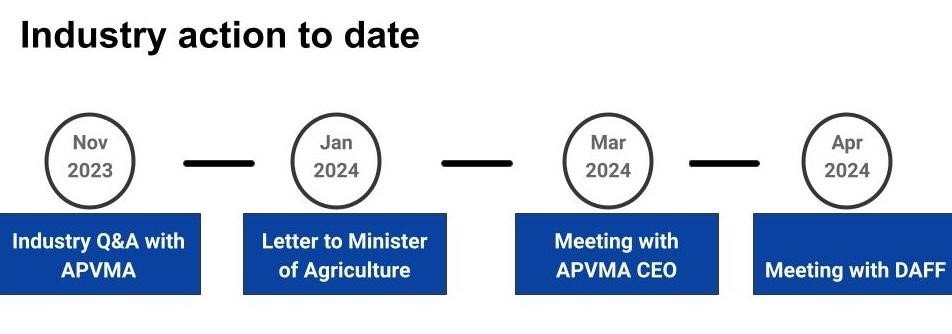
After considerable additional conversation between APVMA, DAFF, and representatives of the Australian Hemp Industry, the APVMA have provided the following pathways to compliance:
- Hemp fractions used for sustenance purposes only (meal and hulls) must undergo a technical assessment by APVMA.
- Hemp fractions used in a product making claims but are not the therapeutic ingredient (e.g. hemp seed oil) must undergo an Ingredient Determination by the APVMA.
- Hemp fractions with intended therapeutic use and claims must be registered with the APVMA as a Veterinary Chemical Product.
All pathways require paying fees to the APVMA. The AHC will be pursuing pathways 1 and 2 for the benefit of the hemp industry as a whole. Combined fees for pathway 1 and 2 could be upwards of $50,000.
Businesses currently selling animal products containing hemp – what to do
Please keep in mind that under APVMA’s current position on hemp, any business selling hemp products for animal consumption is violating the AgVet code and could be penalised. There are several options available for affected businesses.
- Remove products from the market until compliance is attained.
- Remove health claims from labels, websites, and any other marketing material, which will reduce your risk of penalisation. Even after hemp is given GRAS status this action still stands in compliance with current regulations.
- Initiate discussions with APVMA by submitting a form for Pre-Application Assistance (PAA). This demonstrates proactivity, reducing the chance penalty. This advice comes directly from APVMA.
- You can choose to do nothing and watch this space, however, you risk a penalty, particularly if you have claims on labelling and are not compliant in other areas such as following good manufacturing processes.
Next steps
The AHC is now running a fundraising campaign with the aim to raise a minimum of $50,000 to cover APVMA fees and re-open the market for hemp seed in animal feeds.
If you are directly affected by the APVMA’s position on cannabis, we urge you to contribute as this will save your businesses significant costs in the future. Please visit our Paws for Wellness (business) page to pledge your contribution. Donations can also be made directly on our Paws for Wellness (donations) page.
Please share this campaign with your networks and encourage support for this important industry action.
Funds collected will be used to cover APVMA fees for a Technical Assessment for hemp hulls and meal (Pathway 1), and an Ingredient Determination for hemp seed oil (Pathway 2).
All stakeholders are urged to contribute towards this important issue.
Conclusion
At this very moment, as a result of the declaration by the APVMA, no business in Australia is permitted to sell products containing hemp seed oil or hemp seed by-products such as seed hulls and seed cake for consumption by livestock or companion animals. This has blocked access to the lucrative pet food and livestock feed markets and put a number of businesses at risk of insolvency. It also creates issues for processors, including seed cleaning businesses, where they now need to dispose of the by-products by other more expensive means.
The AHC has a pathway and a solution to this most serious problem, but we need the funds to be able to process the APVMA applications on behalf of industry. We ask those who are affected, to please make an investment into this project that will save you significant expenses in the future if you wish to continue selling animal foods containing hemp.
Only by working together can we solve this issue in a quick and cost effective way.
To stay up to date with progress on this topic, please sign up to our newsletter.




 We are grateful to the
We are grateful to the 
 The AIHC was conceived and designed by its founder, Robert Bell, to both add value and to assist in the future expansion of the industrial hemp industry and its community.
The AIHC was conceived and designed by its founder, Robert Bell, to both add value and to assist in the future expansion of the industrial hemp industry and its community.




 Health
Health







 Our Top 5 Cultivars have been developed without genetic modification to grow and thrive in most regions of Australia.
Our Top 5 Cultivars have been developed without genetic modification to grow and thrive in most regions of Australia.

 AVAILABLE PLANTING SEED:
AVAILABLE PLANTING SEED:
 As the Deputy CEO of the Northern Territory Farmers Association (NT Farmers), I am committed to advancing agricultural development in Northern Australia.
As the Deputy CEO of the Northern Territory Farmers Association (NT Farmers), I am committed to advancing agricultural development in Northern Australia.  Jaimie Milling is a fourth-generation farmer who has been in the agriculture industry all his life.
Jaimie Milling is a fourth-generation farmer who has been in the agriculture industry all his life.
